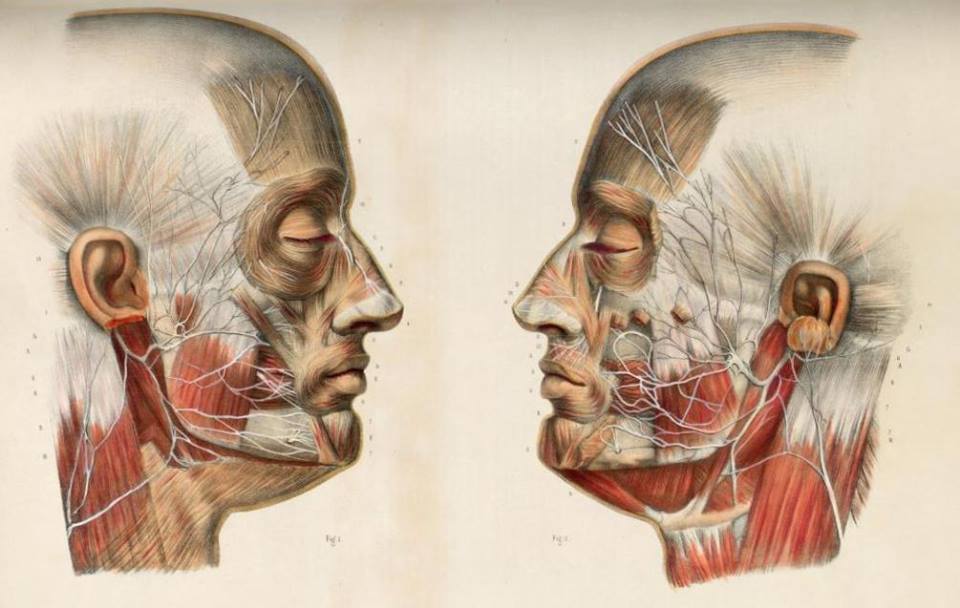
Intraoperative Monitoring of the Facial Nerve
The use of intraoperative monitoring improves postoperative facial nerve (Facial Nerve) function and is an indispensable tool for facilitating surgery involving the Facial Nerve. This tool, however, must be properly applied and maintained, in order to maximize its potential benefits and avoid inconvenient results. To achieve this goal, a thorough understanding of monitoring techniques is required. This chapter will consider the technical aspects of Facial Nerve monitoring. The different techniques used to monitor the Facial Nerve are discussed with emphasis on how to conduct the technique and solve common problems to ensure successful monitoring. The practical aspects of instrumentation, electrode placement, stimulation parameters, types of responses encountered, and artifact identification are also discussed in detail. However, the use and outcome of Facial Nerve monitoring in specific clinical disorders is beyond the scope of this chapter.
Several intraoperative events can pose an increased risk of injury to the Facial Nerve. The nerve could be directly lacerated during dissection or drilling in close proximity to the nerve. The use of electrocautery, laser energy or even a diamond drill producing excessive heat may cause thermal injury to the nerve. Iatrogenic injury can also occur by stretching the nerve during dissection which can interrupt the myelin sheath, cause variable degree of axonal damage, or compromise the blood supply of the nerve. Another mechanism of Facial Nerve injury is compression of the nerve that can cause facial paralyses by entrapment neuropathy (Monsell, 2005).
Many factors can increase the incidence of iatrogenic Facial Nerve injury, including congenital anomalies, revision surgery, tumors, and severe inflammatory processes. These factors may distort normal anatomical relations, or cause the Facial Nerve to be thinned and attenuated thus making dissection around the nerve a more difficult task (May et al., 2000). The lack of an epineural covering in the intracranial segment of the nerve is an additional factor that makes it more vulnerable to injury where even mild stretching may produce significant damage to the nerve. Whatever the mechanism, iatrogenic injury can produce variable effects ranging from mild transient to severe irreversible damage to the Facial Nerve. A mild transient injury may result from interruption of the myelin sheath covering the nerve leading to delayed latency of the recorded compound muscle action potential (CMAP) recorded from the facial muscles. A more severe injury may be due to interruption of the nerve axons and results in decrease of amplitude of the recorded CMAP. The amount of axon loss can be roughly estimated by comparing the amplitude of the potential with a previous baseline value. With axonal damage, the axons distal to the injury will undergo Wallerian degeneration in about 4 days from the time of injury (Preston and Shapiro, 1998). However, intraoperatively, immediately after the injury, the distal axons will still be responsive to electrical stimulation. Therefore, when assessing the integrity of the Facial Nerve after an injurious event, it is essential to ensure that the stimulator is placed proximal to the site of injury.
Technical Considerations for Facial Nerve Monitoring
The instrumentation requirements for Facial Nerve monitoring are basically similar to those used for electrodiagnostic purposes. The equipment should include an isolated electrical stimulator that can be precisely controlled at low levels, differential amplifiers of a good quality, filters to reduce noise, a multichannel oscilloscope to dis- play the signal (at least four-channel), an audio monitor, and the ability to permanently store and printout data. However, to comply with the needs of the operating room, there are certain modifications and different features that are required for intraoperative equipment, especially those involving electrical safety, stimulation parameters, data collection, and display.
Multiple channel systems are recommended as they allow monitoring of multiple divisions of the Facial Nerve independently, as well as other cranial motor nerves if needed. Even in cases where only the Facial Nerve is at risk, the extra channels can be hooked to the contralateral facial muscles to provide a valuable control for nonspecific increases in EMG activity due to light anesthesia or other nonsurgical factors. For more complex surgical cases, where auditory brainstem response (ABR) may be monitored as well as multiple cranial motor nerves, a multichannel system with averaging capabilities should be used. Such systems are now commercially available from various manufacturers. These systems are also adaptable for other types of surgical monitoring (i.e., spinal surgery) by creating appropriate software templates.
Multiple channel systems are recommended as they allow monitoring of multiple divisions of the Facial Nerve independently, as well as other cranial motor nerves if needed. Even in cases where only the Facial Nerve is at risk, the extra channels can be hooked to the contralateral facial muscles to provide a valuable control for nonspecific increases in EMG activity due to light anesthesia or other nonsurgical factors. For more complex surgical cases, where auditory brainstem response (ABR) may be monitored as well as multiple cranial motor nerves, a multichannel system with averaging capabilities should be used. Such systems are now commercially available from various manufacturers. These systems are also adaptable for other types of surgical monitoring (i.e., spinal surgery) by creating appropriate software templates.
Recording Electrodes
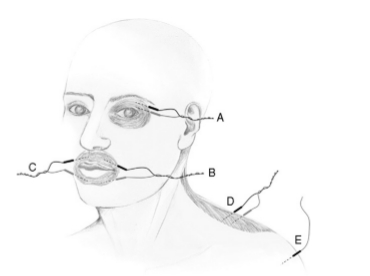
Needle electrodes are preferred to surface electrodes for intraoperative monitoring as they are more specific, less prone to artifacts, and their application is less time consuming than surface electrodes. A variety of different styles of needle electrodes have been used including standard EMG electrodes, subdermal EEG electrodes, and a variety of custom designs. Probably, the most popular recording electrodes for Facial Nerve monitoring are platinum or stainless steel subdermal needles that have a larger uninsulated surface than electrodes designed for single fiber EMG, therefore are more likely to detect activity arising anywhere in the desired muscle. Regardless of the type of electrode used, it is important to insert the electrodes correctly in the desired muscle; this is relatively easy when monitoring the Facial Nerve as facial muscles are superficial and easy to identify (Fig. 1). However, care should be taken in obese patients to keep the needle in the muscle and not to insert it in overlying fat.
After proper insertion, electrodes should be taped in place to avoid accidental dislodgment, as it is usu- ally unfeasible to reach their site of insertion after the patient has been positioned and draped. Electrode leads should be directed away from the surgical field; they may also be loosely taped to the patient’s skin to avoid any accidental movement of the wires into the sterile field or pulling out of the electrode at the insertion site. After the electrodes are secured in place, they are connected to the respective amplifiers which should be already switched on, as electrical surges may develop from switching on the amplifiers and can reach the patient if connected to the amplifier at that time. Impedance of the recording electrodes should always be checked and should be kept below 5 kO (Nuwer et al., 1993). If impedance is high, and/or the recording channel shows increased noise levels, proper electrode insertion and connection to the amplifiers should be verified.
After proper insertion, electrodes should be taped in place to avoid accidental dislodgment, as it is usu- ally unfeasible to reach their site of insertion after the patient has been positioned and draped. Electrode leads should be directed away from the surgical field; they may also be loosely taped to the patient’s skin to avoid any accidental movement of the wires into the sterile field or pulling out of the electrode at the insertion site. After the electrodes are secured in place, they are connected to the respective amplifiers which should be already switched on, as electrical surges may develop from switching on the amplifiers and can reach the patient if connected to the amplifier at that time. Impedance of the recording electrodes should always be checked and should be kept below 5 kO (Nuwer et al., 1993). If impedance is high, and/or the recording channel shows increased noise levels, proper electrode insertion and connection to the amplifiers should be verified.
* In my situation I would monitor all five branches of the facial nerve using the Frontalis, Orbicularis Oculi, Zygomatic Branch, Orbicularis Oris, and the platysma with a grounding needle and free-running EMG during stimulation to identify the branch.*
Stimulating Electrodes
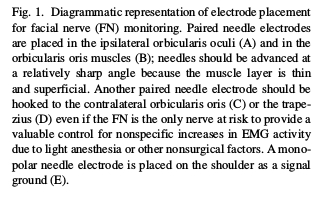
Various designs of stimulating electrodes have been used during Facial Nerve monitoring, each with its advantages and disadvantages. Monopolar electrodes have a single stimulating electrode in the hand piece, the tip of which should be connected to the cathode of the stimulator; the anodal return or reference is usually a needle inserted at the periphery of the wound. Several types of monopolar electrodes have been described, the most commonly used ones are the Prass flush-tip; ball tip, and flexible platinum–iridium tip (Fig. 2). With monopolar electrodes, current from the stimulating electrode travels a longer path through the body tis- sues in order to reach the anodal return which is relatively far away, thus increasing the likelihood of current spread. As a result, nerves in close proximity to each other may be stimulated as a group. It may therefore be more difficult to precisely locate an individual nerve or accurately identify nerve fibers within other tissues when monopolar stimulation is used (Danke and Wiegand, 1994). On the other hand, bipolar electrodes do not use a distant reference; thus current spread is minimized leading to more accurate localization of neural tissue. However, a major limitation to the use of bipolar electrodes is that the response is greatly affected by variations in the orientation of the bipolar tips in relation to the long axis of the nerve (Kartush et al., 1985). Commercially monopolar electrodes are more widely used as they do not have this disadvantage and if the intensity is near the threshold level they can provide spatial resolution of less than 1 mm (Yingling and Ashram, 2005b). To overcome the problem of probe orientation when using bipolar electrodes, Danke and Wiegand (1994) suggested using coaxial bipolar stimulation in which the stimulating electrode is entirely and symmetrically sur- rounded by the reference electrode in a coaxial configuration.
All the previously described probes are used entirely for stimulation, and thus dissection has to stop each time stimulation is used. Kartush et al., (1985) introduced a set of dissecting instruments that can be interchangeably connected to the electrical stimulator, allowing simultaneous dissection with continuous stimulation. These stimulators are mainly useful for removing parts of tumor closely adherent to a nerve.
All the previously described probes are used entirely for stimulation, and thus dissection has to stop each time stimulation is used. Kartush et al., (1985) introduced a set of dissecting instruments that can be interchangeably connected to the electrical stimulator, allowing simultaneous dissection with continuous stimulation. These stimulators are mainly useful for removing parts of tumor closely adherent to a nerve.
Stimulator Parameters
Despite the wide use of intraoperative electrical stimulation of the Facial Nerve, stimulation criteria differ consider- ably among various centers and there is still no consensus about the optimum criteria to be applied. Although concerns have been raised about the safety of direct electrical stimulation of the Facial Nerve, the electrical stimulation parameters provided by commercial equipment for Facial Nerve monitoring are safe if properly utilized. Electrical stimulators in clinical use deliver rectangular pulses, the amplitude and duration of which vary within a wide range. Our preference is to use monopolar constant voltage stimulation, delivering pulses of 0.2 ms duration at a rate of 5–10/s. With these parameters, the threshold for an evoked EMG response from normal nerves is usually between 0.05 and 0.2 V, averaging about 0.1 V (Yingling and Ashram, 2005a).
The issue of whether constant current or constant voltage stimulators should be used has been a source of continuing controversy. There is no literature that demonstrates a clear advantage for either method. Whether constant current or constant voltage is being used in different centers is often determined by the capability of the instrumentation, or with which method the team has most experience and feels most comfortable.
The issue of whether constant current or constant voltage stimulators should be used has been a source of continuing controversy. There is no literature that demonstrates a clear advantage for either method. Whether constant current or constant voltage is being used in different centers is often determined by the capability of the instrumentation, or with which method the team has most experience and feels most comfortable.
Facial Nerve Monitoring Techniques
Several techniques for intraoperative Facial Nerve monitoring have made their way to clinical use, such as intraoperative EMG, recording of compound nerve action potential (CNAP), facial muscle F wave recording, and video monitoring. EMG is by far the most widely used technique, and will be the primary method discussed in this chapter.
Intraoperative EMG Monitoring
There are two major ways in which intraoperative EMG can be used to monitor the Facial Nerve. The first is monitoring mechanically evoked or spontaneous EMG activity. Such activity is continuously recorded to detect thermal mechanical or electrical irritation of the Facial Nerve that can result from intraoperative events. The second method is electrical stimulation of the exposed Facial Nerve and recording an evoked EMG response from the corresponding facial muscles to locate and identify the Facial Nerve.
The term mechanically evoked or spontaneous EMG activity has been used to describe motor unit activity elicited in the facial muscles in response to surgical manipulation of the Facial Nerve. Such activity has also been called neurotonic discharges, which are high frequency bursts of motor unit action potential (MUAP) activity generated in response to mechanical or metabolic irritation of the nerve that innervates the muscle (Harper and Daube, 1998). Electrical stimulation, on the other hand, produces a synchronous composite electrical activity within the target muscle called the CMAP. Measurements of the threshold, latency, and amplitude of the CMAP are the basis of numerous studies investigating the prognostic value of intra- operative Facial Nerve monitoring.
When the Facial Nerve is stimulated, its fibers are not all equally excited at the same intensity of current. The largest diameter fibers are recruited first as they have lower internal resistance (Robinson, 1995). The minimal stimulus intensity required to produce a response is known as threshold. Increasing the stimulus above the threshold will cause recruitment of the smaller diameter fibers (York, 1992), resulting in increase in the amplitude of the CMAP due to activation of more motor units until all muscle fibers have been activated (suprathreshold stimulation). Amplitude of CMAP reflects the total number of muscle fibers that contribute to the potential, provided that they fire synchronously. Intraoperatively, the amplitude of the CMAP is usually measured from the most negative peak to the most positive peak (peak-to-peak amplitude). Decrease of CMAP amplitude primarily results from fewer activated axons, however, it may also result from partial conduction block (Campbell, 1998).
The term mechanically evoked or spontaneous EMG activity has been used to describe motor unit activity elicited in the facial muscles in response to surgical manipulation of the Facial Nerve. Such activity has also been called neurotonic discharges, which are high frequency bursts of motor unit action potential (MUAP) activity generated in response to mechanical or metabolic irritation of the nerve that innervates the muscle (Harper and Daube, 1998). Electrical stimulation, on the other hand, produces a synchronous composite electrical activity within the target muscle called the CMAP. Measurements of the threshold, latency, and amplitude of the CMAP are the basis of numerous studies investigating the prognostic value of intra- operative Facial Nerve monitoring.
When the Facial Nerve is stimulated, its fibers are not all equally excited at the same intensity of current. The largest diameter fibers are recruited first as they have lower internal resistance (Robinson, 1995). The minimal stimulus intensity required to produce a response is known as threshold. Increasing the stimulus above the threshold will cause recruitment of the smaller diameter fibers (York, 1992), resulting in increase in the amplitude of the CMAP due to activation of more motor units until all muscle fibers have been activated (suprathreshold stimulation). Amplitude of CMAP reflects the total number of muscle fibers that contribute to the potential, provided that they fire synchronously. Intraoperatively, the amplitude of the CMAP is usually measured from the most negative peak to the most positive peak (peak-to-peak amplitude). Decrease of CMAP amplitude primarily results from fewer activated axons, however, it may also result from partial conduction block (Campbell, 1998).
Anesthetic Requirements For Intraoperative Neuromonitoring
Intraoperative EMG responses are basically unaffected by routine concentrations of common anesthetics such as nitrous oxide, opiates, or halogenated agents, so there are generally no constraints necessary on anesthetic techniques, except avoiding the use of muscle relaxants, since blockade of the neuromuscular junction interferes with meaningful monitoring of EMG activity (Blair et al., 1994). It is therefore recommended not to use any paralytic agent during Facial Nerve monitoring. Short-acting agents such as succinylcholine may be given at the time of intubation, but it should be confirmed that such agents have cleared before monitoring begins. While muscle relaxants can be useful to facilitate ventilation, prevent patient movement, and allow use of less anesthetic agents, during Facial Nerve monitoring, the anesthesiologist must use other agents to maintain an adequate depth of anesthesia to prevent patient movement.
Use of Electrical Stimulation for Facial Nerve Monitoring
Before using electrical stimulation for Facial Nerve monitoring, it is important to confirm that the stimulating system is functioning, keeping in mind that the presence of a stimulus artifact does not confirm a functioning system. It is possible to have a stimulus artifact with only one lead (either the anodal return or the cathodal stimulator) connected. However, the absence of any artifact usually indicates an open circuit somewhere in the system. To avoid any doubt, it is a good strategy to confirm the operation of the entire system before monitoring begins; a simple way to achieve this is by placing the stimulating electrode directly on a muscle and obtaining a direct muscular response, although muscle requires higher stimulation levels than nerve. Alternately, a different motor nerve can be stimulated; for example, the spinal accessory nerve (XI) can often be accessed during a retrosigmoid craniotomy and used to confirm the integrity of the stimulator by obtaining a CMAP from the trapezius muscle. Once system function has been confirmed, electrical stimulation can be used to confirm the absence of a nerve in a specific location. The various uses of electrical stimulation during Facial Nerve monitoring which go beyond just localizing the course of the nerve will be discussed in the following section.
Use of stimulation to identify and map the course of the Facial Nerve. Facial Nerve identification is not a major problem in cases with relatively undistorted anatomy such as uncomplicated middle ear surgery, microvascular decompression, or vestibular neurectomy where the anatomy of the Facial Nerve and its relations among the various cranial nerves are relatively constant. How- ever, the presence of pathology, such as congenital anomalies or a space-occupying lesion in the temporal bone or posterior fossa, may make identification based on anatomical relationships difficult or impossible. If the nerve can be located at the beginning of surgery, its location can be confirmed by an electrical response before dissection begins. After establishing a threshold for stimulation, the voltage is increased to about three times the threshold (suprathreshold stimulation) and the stimulator swept across the exposed surface for dissection to confirm that there are no Facial Nerve fibers before beginning dissection. If the nerve is visually difficult to identify at the beginning of dissection, the only way to locate and trace the Facial Nerve is with electrical stimulation. A practical strategy is to start mapping the accessible region with an intensity of 0.3 V until a response is obtained and the Facial Nerve is located. If no response is obtained, the search is repeated at 0.5 and 1.0 V. If no response is obtained at 1.0 V, it can be safely assumed that the Facial Nerve is not on the exposed surface and dissection can proceed. (Yingling and Ashram, 2005a).
Use of stimulation to differentiate between the Facial Nerve and other cranial nerves in the cerebellopontine angle. In larger tumors, cranial motor nerves may be displaced in unexpected locations; it may not be possible with visual inspection of the surgical field to confirm which nerve is being encountered. To gain more insight into anatomical relationships, electrical stimulation can be used to distinguish between the Facial Nerve and other cranial nerves such as the trigeminal, vestibulocochlear and to a lesser extent the abducens and lower cranial nerves.
A tumor can push the Facial Nerve rostrally so that it is located very close to the trigeminal nerve. Stimulation of the motor fibers of the trigeminal nerve produces EMG responses in the masseter and temporalis muscles, and responses in these larger muscles can produce a volume conducted response on Facial Nerve monitoring.
channels. However, these two nerves can be differentiated from one another despite overlap in the responding channels by their different onset latencies. Stimulation of the trigeminal nerve produces EMG responses that are of a considerably shorter latency (3–4ms to onset) than those to Facial Nerve stimulation (6–8 ms), allowing these nerves to be distinguished (Moller, 1995).
Similarly, the vestibulocochlear nerve has an anatomically close relation to the Facial Nerve in the cerebello- pontine angle (CPA) and internal auditory canal, and confirmation of the identity of the nerve is some- times essential before manipulation. By electrical stimulation with an intensity just above threshold, a response is produced on the Facial Nerve monitoring channels only to direct Facial Nerve stimulation. If the vestibuloco- chlear nerve is stimulated, no response is produced in facial muscles below $0.7 V, much above the threshold for FN stimulation (Ashram et al., 2005).
25.3.1.3.3. The use of stimulation before bipolar electrocoagulation to confirm that the area to be cauterized is free from FN fibers. Although bipolar cautery produces less spread of electrical current compared to monopolar cautery, neural injury can still occur owing to spread of heat by convection or conduction (Kartush and Bouchard, 1992). There- fore, the Facial Nerve is at a significant risk of thermal injury during electrocautery. Furthermore, bipolar devices generate a high amplitude broad band noise that is difficult to filter, thus rendering EMG monitoring useless during a time when the Facial Nerve is potentially at high risk. In our experience, a practical way to deal with this problem is to confirm that the nerve is not in an area about to be cauterized, by using higher levels of electrical stimulation (up to 1 V). If no response is obtained at this level, it can be assumed that electrocautery can proceed safely.
25.3.1.3.4. Assessment of functional status of the Facial Nerve and prediction of postoperative outcome by electrical stimulation. Another important utility of electrical stimulation is the determination of the functional status of the Facial Nerve at the end of dissection and prediction of postoperative functional outcome. Several groups of investigators have studied the prognostic value of intraoperative electrical stimulation in predicting postoperative Facial Nerve outcome using different parameters based on measurement of the CMAP. These methods include measurement of the threshold of Facial Nerve stimulation at the end of dissection proximal to the tumor at the brainstem (Lalwani et al., 1994; Silverstein et al., 1994; Zeitouni et al., 1997), as well as measurement of the peak-to-peak amplitude of the CMAP at both the orbicularis oculi and orbicularis oris muscles (Mandpe et al., 1998). Other methods based on pre- and postresection comparisons of thresholds (Prasad et al., 1993), or amplitudes (Ebersold et al., 1992; Taha et al., 1995; Mandpe et al., 1998) have also been suggested. Unfortunately, there is still no agreement about which method provides the highest degree of accuracy and sensitivity. The best intraoperative predictor of postoperative outcome remains to be determined. A detailed discussion of the prognostic significance of each of these methods is beyond the scope of this chapter.
A tumor can push the Facial Nerve rostrally so that it is located very close to the trigeminal nerve. Stimulation of the motor fibers of the trigeminal nerve produces EMG responses in the masseter and temporalis muscles, and responses in these larger muscles can produce a volume conducted response on Facial Nerve monitoring.
channels. However, these two nerves can be differentiated from one another despite overlap in the responding channels by their different onset latencies. Stimulation of the trigeminal nerve produces EMG responses that are of a considerably shorter latency (3–4ms to onset) than those to Facial Nerve stimulation (6–8 ms), allowing these nerves to be distinguished (Moller, 1995).
Similarly, the vestibulocochlear nerve has an anatomically close relation to the Facial Nerve in the cerebello- pontine angle (CPA) and internal auditory canal, and confirmation of the identity of the nerve is some- times essential before manipulation. By electrical stimulation with an intensity just above threshold, a response is produced on the Facial Nerve monitoring channels only to direct Facial Nerve stimulation. If the vestibuloco- chlear nerve is stimulated, no response is produced in facial muscles below $0.7 V, much above the threshold for FN stimulation (Ashram et al., 2005).
25.3.1.3.3. The use of stimulation before bipolar electrocoagulation to confirm that the area to be cauterized is free from FN fibers. Although bipolar cautery produces less spread of electrical current compared to monopolar cautery, neural injury can still occur owing to spread of heat by convection or conduction (Kartush and Bouchard, 1992). There- fore, the Facial Nerve is at a significant risk of thermal injury during electrocautery. Furthermore, bipolar devices generate a high amplitude broad band noise that is difficult to filter, thus rendering EMG monitoring useless during a time when the Facial Nerve is potentially at high risk. In our experience, a practical way to deal with this problem is to confirm that the nerve is not in an area about to be cauterized, by using higher levels of electrical stimulation (up to 1 V). If no response is obtained at this level, it can be assumed that electrocautery can proceed safely.
25.3.1.3.4. Assessment of functional status of the Facial Nerve and prediction of postoperative outcome by electrical stimulation. Another important utility of electrical stimulation is the determination of the functional status of the Facial Nerve at the end of dissection and prediction of postoperative functional outcome. Several groups of investigators have studied the prognostic value of intraoperative electrical stimulation in predicting postoperative Facial Nerve outcome using different parameters based on measurement of the CMAP. These methods include measurement of the threshold of Facial Nerve stimulation at the end of dissection proximal to the tumor at the brainstem (Lalwani et al., 1994; Silverstein et al., 1994; Zeitouni et al., 1997), as well as measurement of the peak-to-peak amplitude of the CMAP at both the orbicularis oculi and orbicularis oris muscles (Mandpe et al., 1998). Other methods based on pre- and postresection comparisons of thresholds (Prasad et al., 1993), or amplitudes (Ebersold et al., 1992; Taha et al., 1995; Mandpe et al., 1998) have also been suggested. Unfortunately, there is still no agreement about which method provides the highest degree of accuracy and sensitivity. The best intraoperative predictor of postoperative outcome remains to be determined. A detailed discussion of the prognostic significance of each of these methods is beyond the scope of this chapter.
25.3.1.3.5. The use of stimulation to assess the integrity of the Facial Nerve after an episode of spontaneous activity and during dissection of large tumors. Electrical stimulation can be used to evaluate the integrity of the Facial Nerve after the occurrence of an alarming episode of spontaneous activity during dissection. This evaluation requires a baseline measurement of threshold and amplitude proximal to the site of dissection. A change of threshold or amplitude from baseline after an episode of spontaneous activity is an indication that manipulation has caused a change in the functional status of the nerve. Although proximal thresh- old and amplitude measurement at the beginning of surgery may not always be possible to obtain in large tumors, it should be attempted as they provide an idea about the baseline values.
The use of electrical stimulation during dissection of large CPA tumors is an important adjunct to spontaneous EMG to give information about the condition of the nerve. In large tumors, axons of the Facial Nerve can become significantly stretched and partially dam- aged, and thus be less likely to respond than healthy ones, producing little EMG when manipulated (Moller, 1995). In such cases, the absence of spontaneous EMG activity should not be relied upon as an indication of lack of mechanical irritation to the Facial Nerve during dissection. Rather, electrical stimulation of the nerve should be used to assess its functional status by comparing responses with a previously recorded baseline (Yingling and Ashram, 2005a).
25.3.1.3.6. Assessment of the thickness of bone over the Facial Nerve during drilling. Electrical stimulation can be used to estimate the thickness of the remaining bone under the burr end. This is important during
the last stages of drilling of the fallopian canal or the internal auditory canal; a decreased threshold (<0.5 V) implies a closer FN to the burr and drilling should be done with marked caution to avoid injury to the underlying FN.
25.3.1.4. Monitoring of spontaneous and mechanically elicited activity
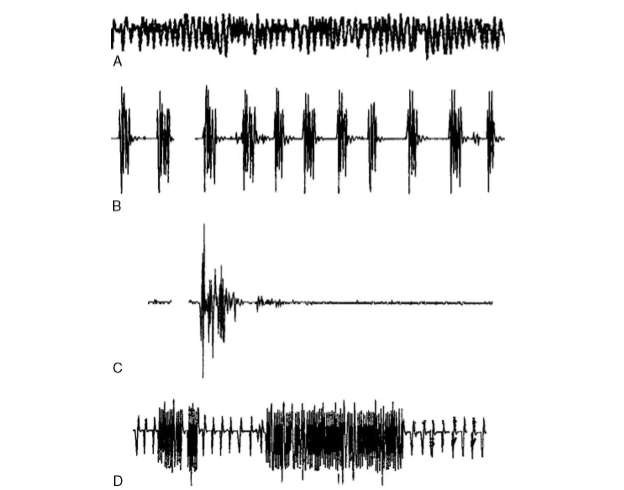
25.3.1.4.1. Patterns of mechanically evoked EMG activity. Almost all patients exhibit some mechanically evoked facial EMG activity during tumor dis- section, retraction, irrigation, or other intraoperative events. Prass and Lu ̈ders (1986) distinguished two types of such EMG activity. They suggested that the phasic “burst” pattern, characterized by short, relatively synchronous bursts of motor unit potentials, corresponded to a single discharge of multiple Facial Nerve axons. This type of activity was associated with direct mechanical nerve trauma, especially blunt trauma, irrigation, or electrocautery. The second pat- tern, tonic or “train” activity, consisted of episodes of prolonged asynchronous grouped motor unit dis- charges which could last up to several minutes. These were most commonly associated with Facial Nerve traction. They further divided such train activity into higher frequency trains (50–100 Hz), called “bomber potentials” due to their sound characteristics, and lower frequency discharges (1–50 Hz), which were more irregular and had a sound resembling popping pop- corn. More recently, Romstock et al. (2000) classified train activity into three distinct patterns A, B, and C trains. A trains are characterized by a sinusoidal symmetrical sequence of high frequency and low amplitude signals that have a sudden onset; B trains are regular or irregular sequences of repeated spikes or bursts with maximum intervals of 500 ms; and C trains are characterized by continuous irregular EMG responses that have many overlapping components. The authors suggested that the occurrence of “A trains” poses an increased risk on the Facial Nerve. Nakao et al. (2001) classified train activity that occurred during the last stage of tumor resection into an irritable pattern with frequent EMG responses to the slightest stimuli; a silent pattern with few or no EMG responses; a stray pattern with persistent train responses up to 20 min despite temporary discontinuance of surgical manipulation; and an ordinary pattern related to mechanical stimulation of the nerve but not easily elicited. They found that the occurrence of silent or stray EMG patterns may be associated with an increased risk of injury to the Facial Nerve.
Fig. 3 shows representative samples of different types of EMG patterns often encountered during Facial Nerve monitoring.
The use of electrical stimulation during dissection of large CPA tumors is an important adjunct to spontaneous EMG to give information about the condition of the nerve. In large tumors, axons of the Facial Nerve can become significantly stretched and partially dam- aged, and thus be less likely to respond than healthy ones, producing little EMG when manipulated (Moller, 1995). In such cases, the absence of spontaneous EMG activity should not be relied upon as an indication of lack of mechanical irritation to the Facial Nerve during dissection. Rather, electrical stimulation of the nerve should be used to assess its functional status by comparing responses with a previously recorded baseline (Yingling and Ashram, 2005a).
25.3.1.3.6. Assessment of the thickness of bone over the Facial Nerve during drilling. Electrical stimulation can be used to estimate the thickness of the remaining bone under the burr end. This is important during
the last stages of drilling of the fallopian canal or the internal auditory canal; a decreased threshold (<0.5 V) implies a closer FN to the burr and drilling should be done with marked caution to avoid injury to the underlying FN.
25.3.1.4. Monitoring of spontaneous and mechanically elicited activity
25.3.1.4.1. Patterns of mechanically evoked EMG activity. Almost all patients exhibit some mechanically evoked facial EMG activity during tumor dis- section, retraction, irrigation, or other intraoperative events. Prass and Lu ̈ders (1986) distinguished two types of such EMG activity. They suggested that the phasic “burst” pattern, characterized by short, relatively synchronous bursts of motor unit potentials, corresponded to a single discharge of multiple Facial Nerve axons. This type of activity was associated with direct mechanical nerve trauma, especially blunt trauma, irrigation, or electrocautery. The second pat- tern, tonic or “train” activity, consisted of episodes of prolonged asynchronous grouped motor unit dis- charges which could last up to several minutes. These were most commonly associated with Facial Nerve traction. They further divided such train activity into higher frequency trains (50–100 Hz), called “bomber potentials” due to their sound characteristics, and lower frequency discharges (1–50 Hz), which were more irregular and had a sound resembling popping pop- corn. More recently, Romstock et al. (2000) classified train activity into three distinct patterns A, B, and C trains. A trains are characterized by a sinusoidal symmetrical sequence of high frequency and low amplitude signals that have a sudden onset; B trains are regular or irregular sequences of repeated spikes or bursts with maximum intervals of 500 ms; and C trains are characterized by continuous irregular EMG responses that have many overlapping components. The authors suggested that the occurrence of “A trains” poses an increased risk on the Facial Nerve. Nakao et al. (2001) classified train activity that occurred during the last stage of tumor resection into an irritable pattern with frequent EMG responses to the slightest stimuli; a silent pattern with few or no EMG responses; a stray pattern with persistent train responses up to 20 min despite temporary discontinuance of surgical manipulation; and an ordinary pattern related to mechanical stimulation of the nerve but not easily elicited. They found that the occurrence of silent or stray EMG patterns may be associated with an increased risk of injury to the Facial Nerve.
Fig. 3 shows representative samples of different types of EMG patterns often encountered during Facial Nerve monitoring.
|
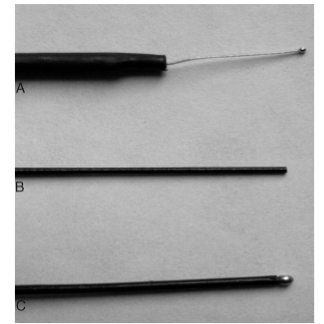
Fig. 3. Examples of EMG activity often seen during facial nerve (Facial Nerve) monitoring. A: Dense tonic (sustained) train activity, having a sinusoidal pattern, usually occurring with nerve stretch. B: Lower frequency sustained activity called popcorn activity. C: Burst activity which is a phasic (transient) activity that is usually associated with direct contact with the nerve. Such events are not of major significance unless they are of large amplitude and occur during critical stages of dissection. D: Burst activity superimposed on ongoing small-amplitude train. Such events overlapping on background activity should be identified because they may pass unnoticed despite their significance (reprinted from Yingling and Ashram, 2005a with permission from Lippincott, Williams and Wilkins).
|
25.3.1.4.2. Recording and interpretation of spontaneous EMG. The occurrence of burst activity during surgery is due to direct mechanical manipulation, usually from blunt contact with the nerve. Their occurrence is a warning that the Facial Nerve was being stimulated enough to result in depolarization and production of facial muscle EMG response but not necessarily enough to be injured (Prass et al., 1987). Accordingly, the occurrence of small amplitude bursts (<500mV in amplitude) is not of major
concern and does not usually mean an increased risk of injury to the Facial Nerve (Magliulo and Zardo, 1997; Romstock et al., 2000). The surgeon should not be warned each time such activity occurs, although in some situations their occurrence may be of utility to the surgeon. Hearing such activity through the loud speaker during dissection is of importance to the surgeon as it develops a high index of anticipation for their occurrence, helping develop safer surgical techniques for dissection (Prass, 1996). They may also be the first indicator to the proximity of the Facial Nerve at the beginning of dissection or during drilling of bone over the nerve. During initial drilling, there is a layer of bone overlying the Facial Nerve but at the final stage of drilling, the bone layer is markedly thinned out and the possibility of burst activity being due to direct contact with the nerve is increased. Therefore, bursts occurring at this stage may be an indication of impending direct contact with the Facial Nerve and require more cautious drilling to avoid serious impact with the Facial Nerve fibers. This can be confirmed as previously mentioned by electrical stimulation and by noting a decrease in threshold of Facial Nerve stimulation from a previous baseline.
On the other hand, when burst activity of large amplitude (500 mV or more) occurs during dissection or final stages of drilling, the surgeon should be promptly warned. He may decide to alter the technique or move to another area of dissection, as such activity may indicate a degree of Facial Nerve injury, the extent of which varies according to the force and number of impacts (Yingling and Ashram, 2005a)
Train activity during Facial Nerve monitoring can occur due to traction compression, contusion, heat spread by electrocoagulation, or saline irrigation to the Facial Nerve. The neurophysiologist performing monitoring should know when to warn the surgeon and not to alert the surgeon each time such activity is displayed, as this may interrupt rather than help surgery and the surgeon is likely to lose confidence in the monitoring team. In general, there are several factors to be considered when interpreting train activity. First, it should be interpreted in relation to a surgical event whenever possible; for example, if train activity occurs after saline irrigation there is no reason to raise concern. However, if it occurs due to traction or dissection in close proximity to the Facial Nerve, the surgeon should be warned. It is worth noting here that sometimes train activity starts with no apparent reason, or may be observed in baseline recordings and decrease as the nerve is decompressed with opening of the dura and draining of Cerebro Spinal Fluid. Although worrisome to the surgeon, such activity does not usually pose a significant risk to the Facial Nerve.
A second point to consider is amplitude of train activity. Small amplitude train activity does not usually mean an impending injury to the nerve; its occurrence, however, does indicate the proximity of the Facial Nerve to the region of dissection and caution should therefore be taken. It could also be considered a good sign, since its presence implies an intact and responsive nerve (Prass et al., 1987). On the other hand, when train activity of large amplitude (>500 mV) occurs during dissection around the nerve, the surgeon should be warned immediately because such activity may be associated with a degree of nerve injury (Beck et al., 1991; Nakao et al., 2001). However, the absence of train activity of large amplitude does not necessarily imply safer dissection, especially in large tumors in which Facial Nerve fibers become significantly compressed. Furthermore, in some cases, a change of pattern of EMG from a responsive to a silent one can occur, in which a nerve that was readily responsive to various mechanical stimuli in early stages of dissection becomes less responsive towards the final stages of resection. Spontaneous activity becomes less frequent and of low amplitude despite significant surgical manipulation of the Facial Nerve. This decline in spontaneous activity may give a false sense of security, causing more vigorous dissection with the possibility of permanent damage to the nerve. Therefore, such a change of pattern should be cautiously interpreted and considered an ominous sign indicating a certain degree of nerve injury has already occurred.
A third point to consider when interpreting train activity is the pattern of activity; certain patterns of train activity have been suggested to pose a risk of injury to the Facial Nerve, such as the type A trains, stray pat- tern, and bomber-type high-frequency trains described above. Fourth, ongoing EMG activity is often an indirect indicator of depth of anesthesia, which is of particular concern when no muscle relaxants are used. A simultaneous increase in spontaneous EMG activity on all channels is unlikely to result from localized dis- section. When such a generalized increase occurs, the anesthesiologist should be notified immediately to increase the depth of anesthesia and avoid overt patient movement.
In conclusion, the analysis of spontaneous EMG activity is not based on a single criterion but rather a combination of factors that together lead to a correct interpretation, including surgical orientation, awareness of surgical events and anesthetic conditions, and understanding of waveform configuration and morphology.
concern and does not usually mean an increased risk of injury to the Facial Nerve (Magliulo and Zardo, 1997; Romstock et al., 2000). The surgeon should not be warned each time such activity occurs, although in some situations their occurrence may be of utility to the surgeon. Hearing such activity through the loud speaker during dissection is of importance to the surgeon as it develops a high index of anticipation for their occurrence, helping develop safer surgical techniques for dissection (Prass, 1996). They may also be the first indicator to the proximity of the Facial Nerve at the beginning of dissection or during drilling of bone over the nerve. During initial drilling, there is a layer of bone overlying the Facial Nerve but at the final stage of drilling, the bone layer is markedly thinned out and the possibility of burst activity being due to direct contact with the nerve is increased. Therefore, bursts occurring at this stage may be an indication of impending direct contact with the Facial Nerve and require more cautious drilling to avoid serious impact with the Facial Nerve fibers. This can be confirmed as previously mentioned by electrical stimulation and by noting a decrease in threshold of Facial Nerve stimulation from a previous baseline.
On the other hand, when burst activity of large amplitude (500 mV or more) occurs during dissection or final stages of drilling, the surgeon should be promptly warned. He may decide to alter the technique or move to another area of dissection, as such activity may indicate a degree of Facial Nerve injury, the extent of which varies according to the force and number of impacts (Yingling and Ashram, 2005a)
Train activity during Facial Nerve monitoring can occur due to traction compression, contusion, heat spread by electrocoagulation, or saline irrigation to the Facial Nerve. The neurophysiologist performing monitoring should know when to warn the surgeon and not to alert the surgeon each time such activity is displayed, as this may interrupt rather than help surgery and the surgeon is likely to lose confidence in the monitoring team. In general, there are several factors to be considered when interpreting train activity. First, it should be interpreted in relation to a surgical event whenever possible; for example, if train activity occurs after saline irrigation there is no reason to raise concern. However, if it occurs due to traction or dissection in close proximity to the Facial Nerve, the surgeon should be warned. It is worth noting here that sometimes train activity starts with no apparent reason, or may be observed in baseline recordings and decrease as the nerve is decompressed with opening of the dura and draining of Cerebro Spinal Fluid. Although worrisome to the surgeon, such activity does not usually pose a significant risk to the Facial Nerve.
A second point to consider is amplitude of train activity. Small amplitude train activity does not usually mean an impending injury to the nerve; its occurrence, however, does indicate the proximity of the Facial Nerve to the region of dissection and caution should therefore be taken. It could also be considered a good sign, since its presence implies an intact and responsive nerve (Prass et al., 1987). On the other hand, when train activity of large amplitude (>500 mV) occurs during dissection around the nerve, the surgeon should be warned immediately because such activity may be associated with a degree of nerve injury (Beck et al., 1991; Nakao et al., 2001). However, the absence of train activity of large amplitude does not necessarily imply safer dissection, especially in large tumors in which Facial Nerve fibers become significantly compressed. Furthermore, in some cases, a change of pattern of EMG from a responsive to a silent one can occur, in which a nerve that was readily responsive to various mechanical stimuli in early stages of dissection becomes less responsive towards the final stages of resection. Spontaneous activity becomes less frequent and of low amplitude despite significant surgical manipulation of the Facial Nerve. This decline in spontaneous activity may give a false sense of security, causing more vigorous dissection with the possibility of permanent damage to the nerve. Therefore, such a change of pattern should be cautiously interpreted and considered an ominous sign indicating a certain degree of nerve injury has already occurred.
A third point to consider when interpreting train activity is the pattern of activity; certain patterns of train activity have been suggested to pose a risk of injury to the Facial Nerve, such as the type A trains, stray pat- tern, and bomber-type high-frequency trains described above. Fourth, ongoing EMG activity is often an indirect indicator of depth of anesthesia, which is of particular concern when no muscle relaxants are used. A simultaneous increase in spontaneous EMG activity on all channels is unlikely to result from localized dis- section. When such a generalized increase occurs, the anesthesiologist should be notified immediately to increase the depth of anesthesia and avoid overt patient movement.
In conclusion, the analysis of spontaneous EMG activity is not based on a single criterion but rather a combination of factors that together lead to a correct interpretation, including surgical orientation, awareness of surgical events and anesthetic conditions, and understanding of waveform configuration and morphology.
Limitations and pitfalls when using EMG
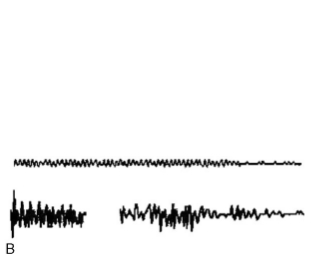
Despite the wide use of intraoperative EMG monitoring, it still has its limitations. A major drawback is that EMG channels can easily pick up artifacts from numerous operating room sources. It is important to distinguish artifact from true EMG to avoid false positive alarms which can be misleading to the surgeon. Such distinction may sometimes be difficult and requires experienced monitoring personnel. There are several causes of intraoperative artifact; some artifacts are easily identified as linked to the use of electrocautery equipment, ultrasonic aspirators, lasers, drills, etc. Other artifacts are more mis- leading, such as those due to contact between surgical instruments made of different metals (bimetallic potentials); these may be associated with similar intraoperative events as those producing true EMG responses. Some useful hints to distinguish artifact from EMG is that artifacts tend to appear simultaneously on several channels, which is unlikely for EMG. Artifacts are usually higher in frequency content than EMG, and thus have a “crackly” sound while true EMG, has more of a “popping” sound on the loud speaker. Another hint is that artifacts tend to be regular in morphology while physiological waves have a more variable morphology (Fig. 4).
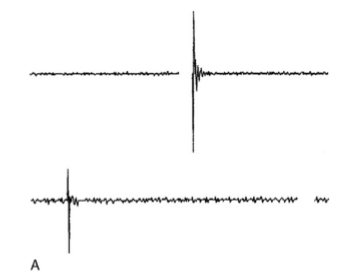
Another problem with EMG is that Facial Nerve stimulation cannot be performed unless the nerve is accessible in the surgical field. However, when removing large tumors the Facial Nerve is at risk of being traumatized before it is visually apparent to the surgeon, and may not be responsive to mechanical manipulation. In this situation, the Facial Nerve location can be anticipated by using a flexible-tip probe to stimulate within dissection planes or even behind the tumor, out of the surgeon’s view, without concern for damaging unseen vascular or neural structures (Yingling and Gardi, 1992). The importance of localizing the Facial Nerve at the start of dissection is emphasized by the fact that a sharp cut to the Facial Nerve will elicit no EMG activity and may pass unrecognized; furthermore, the distal segment of a transected nerve may still give a response to both mechanical and electrical stimulation, giving a false sense of security (Fig. 5).
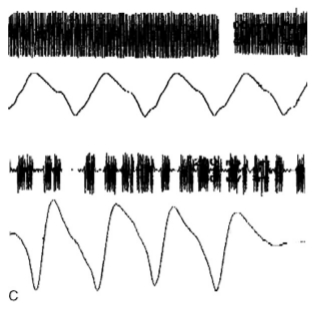
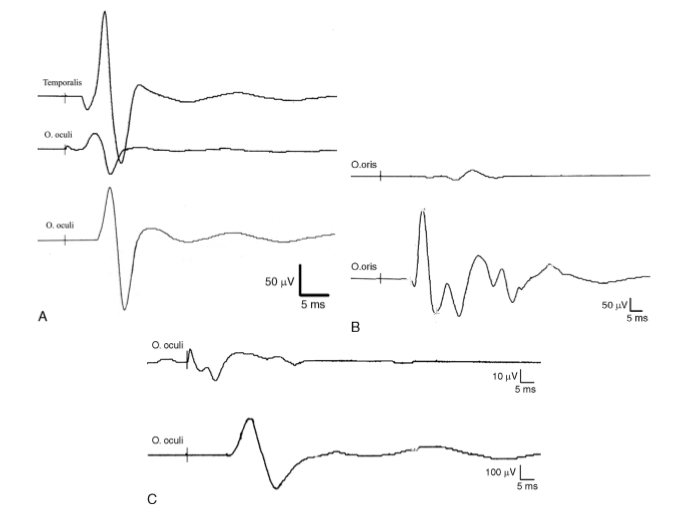
A potentially misleading pitfall when performing electrical stimulation is a volume conducted response on a Facial Nerve monitoring channel after stimulation of another cranial nerve. For example, stimulation of the trigeminal nerve may give a response on Facial Nerve monitoring channels and may be mistaken for the Facial Nerve; however, the two nerves can be identified by their different latencies as mentioned previously. This is facilitated by the use of multichannel monitoring with separate channels for recording EMG responses from different cranial nerves (Fig. 6). Another example, is stimulation of the nervus intermedius which may be mistaken for a thinned attenuated Facial Nerve filament in the CPA. Stimulation of the nervus intermedius can cause volume conducted EMG activity in the Facial Nerve monitoring channels (at least the orbicularis oris), but the main trunk of the Facial Nerve may lie in an entirely different location within the CPA. The nervus intermedius response can be recognized by its electrophysiological features (Ashram et al., 2005). Once the nervus intermedius is recognized, it is crucial for the surgeon to locate the main trunk of the Facial Nerve by further stimulation mapping.
Despite the wide use of intraoperative EMG monitoring, it still has its limitations. A major drawback is that EMG channels can easily pick up artifacts from numerous operating room sources. It is important to distinguish artifact from true EMG to avoid false positive alarms which can be misleading to the surgeon. Such distinction may sometimes be difficult and requires experienced monitoring personnel. There are several causes of intraoperative artifact; some artifacts are easily identified as linked to the use of electrocautery equipment, ultrasonic aspirators, lasers, drills, etc. Other artifacts are more mis- leading, such as those due to contact between surgical instruments made of different metals (bimetallic potentials); these may be associated with similar intraoperative events as those producing true EMG responses. Some useful hints to distinguish artifact from EMG is that artifacts tend to appear simultaneously on several channels, which is unlikely for EMG. Artifacts are usually higher in frequency content than EMG, and thus have a “crackly” sound while true EMG, has more of a “popping” sound on the loud speaker. Another hint is that artifacts tend to be regular in morphology while physiological waves have a more variable morphology (Fig. 4).
Another problem with EMG is that Facial Nerve stimulation cannot be performed unless the nerve is accessible in the surgical field. However, when removing large tumors the Facial Nerve is at risk of being traumatized before it is visually apparent to the surgeon, and may not be responsive to mechanical manipulation. In this situation, the Facial Nerve location can be anticipated by using a flexible-tip probe to stimulate within dissection planes or even behind the tumor, out of the surgeon’s view, without concern for damaging unseen vascular or neural structures (Yingling and Gardi, 1992). The importance of localizing the Facial Nerve at the start of dissection is emphasized by the fact that a sharp cut to the Facial Nerve will elicit no EMG activity and may pass unrecognized; furthermore, the distal segment of a transected nerve may still give a response to both mechanical and electrical stimulation, giving a false sense of security (Fig. 5).
A potentially misleading pitfall when performing electrical stimulation is a volume conducted response on a Facial Nerve monitoring channel after stimulation of another cranial nerve. For example, stimulation of the trigeminal nerve may give a response on Facial Nerve monitoring channels and may be mistaken for the Facial Nerve; however, the two nerves can be identified by their different latencies as mentioned previously. This is facilitated by the use of multichannel monitoring with separate channels for recording EMG responses from different cranial nerves (Fig. 6). Another example, is stimulation of the nervus intermedius which may be mistaken for a thinned attenuated Facial Nerve filament in the CPA. Stimulation of the nervus intermedius can cause volume conducted EMG activity in the Facial Nerve monitoring channels (at least the orbicularis oris), but the main trunk of the Facial Nerve may lie in an entirely different location within the CPA. The nervus intermedius response can be recognized by its electrophysiological features (Ashram et al., 2005). Once the nervus intermedius is recognized, it is crucial for the surgeon to locate the main trunk of the Facial Nerve by further stimulation mapping.
Fig. 4. Illustrative examples of artifacts that can be mistaken for EMG. A: Upper: bimetallic artifact produced by contact of different metallic instruments in the surgical field (may be confused with spike activity). Note the sharp edge on waveforms with exponential decay. Lower: single EMG spike superimposed on a background of low- amplitude EMG activity and no exponential decay. B: Upper: regular sinusoidal artifact produced during drilling the internal auditory canal (IAC). Lower: irregular EMG activity occurring while drilling the IAC. C: Upper two traces: regular artifact represented on two time scales, 200 and 5 ms/cm, respectively. Bottom two traces: EMG on the same two time scales. At 200 ms/cm, it can be difficult to differentiate between true EMG activity and artifact. However, with the faster 5 ms/cm time base, trace 2 shows that the artifact waveform is regular and synchronized while trace 4 reveals the irregularities that characterize true EMG activity (reprinted from Yingling and Ashram, 2005a with permission from Lippincott, Williams and Wilkins).
|
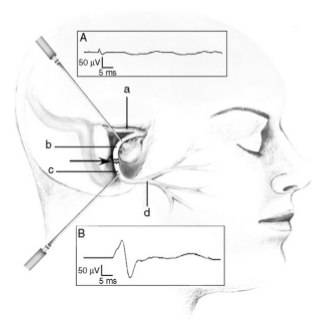
Fig. 5. Diagrammatic representation of site of stimulation of an injured facial nerve (Facial Nerve). Immediately after intra- operative injury to the Facial Nerve, stimulation of the nerve proximal to the site of injury reveals no response (A) (or reduced response if injury is incomplete), while stimulation of the nerve distal to the site of injury results in a normal EMG response (B), therefore it should be confirmed that the stimulator is placed proximal to the site of injury when assessing Facial Nerve function. (a) Labyrinthine segment of the Facial Nerve; (b) tympanic segment of the Facial Nerve proximal to the site of injury; (c) mastoid segment of the Facial Nerve distal to the site of injury; (d) extracranial segment of the Facial Nerve. Arrow points to the site of Facial Nerve injury.
|
Other Modalities used for Facial Nerve Monitoring

The earliest techniques of Facial Nerve monitoring involved stimulation of the Facial Nerve intracranially and watching the patient’s face under the drapes for visible contractions (Krause, 1912; Olivecrona, 1940; Pool, 1966). These old visual methods were revived with the introduction of the new technology of video analysis to detect facial movements (Filipo et al., 2000, 2002). Such techniques assessed facial movements by measuring the shift along a line from labial commissure to zygomatic bone since contraction of the zygomatic muscle moves the labial commissure up and down. Filipo et al. (2002) also compared video monitoring to EMG and found that video monitoring, although less sensitive than EMG, is a reliable method with the advantages of being noninvasive, easy and quick to set up, and more specific than EMG as it only responds to muscle con- traction while EMG may produce many false positive results unless the neurophysiologist is skilled at distinguishing artifacts from true EMG activity.
Another technique that has been used for Facial Nerve monitoring is recording of the CNAP by stimulating the distal Facial Nerve and recording the CNAP from the nerve within the surgical field opposite to the normal direction of impulse conduction (antidromic recording) using a bipolar electrode (Richmond and Mahla, 1985). This technique was further updated by Colletti et al. (1996), using low-intensity stimulation of the mandibular branch of the Facial Nerve and monopolar recording techniques with an intracranial electrode. Methods based on CNAP rather than EMG recording have the advantage that they can be used even if muscle relaxants are used. Another advantage is that these methods allow actual continuous monitoring of Facial Nerve function, in contrast to EMG, which provides information only when the Facial Nerve is mechanically or electrically stimulated. On the other hand, the CNAP monitoring is less sensitive than EMG, and frequently requires averaging. Also, direct nerve monitoring is technically more difficult than EMG; attaching electrodes to delicate neural structures can be difficult and even injurious to the nerve. In addition, electrodes in the surgical field may limit exposure (Colletti et al., 1997).
Recording of F-waves from the nasal muscle is another method that made its way to clinical use in Facial Nerve monitoring (Wedekind and Klug, 1996). The F-wave is a late muscle potential that is believed to be due to recurrent discharge of a small percentage
($1–5%) of the motor neurons. It occurs due to antidromic spread of excitation that reaches the motor neurons and then again propagates orthodromically, producing a delayed muscle contraction. The intra- operative recording of an F-wave should reflect the integrity of the Facial Nerve pathway from the axon hillock to the motor end plate; a stable F-wave or reversible changes that occur during surgery usually indicate good postoperative Facial Nerve function (Wedekind and Klug, 2001). Although the F-wave may be a promising tool for intraoperative Facial Nerve monitoring, its use has been limited by its sensitivity to anesthesia and the fact that it may be normally absent in healthy adults.
Transcranial motor evoked potentials (tcMEP) is a new technique now widely used in spinal surgery. It is performed by electrical stimulation of the motor cortex with recording of EMG responses from appropriate muscles. This technique has been successfully adopted to monitor the Facial Nerve by multipulse stimulation of the contralateral motor cortex and recording of facial MEPs from the orbicularis oris muscle on the operative side. This method can provide an ongoing assessment of Facial Nerve function without the need of the surgeon to stimulate the nerve, and could be especially useful in cases in which the proximal FN is inaccessible to direct stimulation (Dong et al., 2005).
Another method under trial is the blink reflex, which is elicited by stimulation of the supraorbital nerve (trigeminal afferents) and consists of an early ipsilateral EMG response (R1) and a later bilateral response (R2), both mediated by the Facial Nerve (Kimura, 1989). It is the R1 response that is recorded intraoperatively. A main problem with blink reflex, how- ever, is the suppression of reflex activity by general anesthetics. It is possible to overcome this problem by greater reliance on intravenous anesthetics such as propofol together with the use of repetitive train stimulation. A chief advantage of the last two techniques is the ability to continuously monitor the Facial Nerve without the need for the surgeon to directly stimulate the nerve. Further improvement in Facial Nerve monitoring techniques are likely to come about when such methods of continuous assessment of Facial Nerve integrity become more refined and integrated.
Another technique that has been used for Facial Nerve monitoring is recording of the CNAP by stimulating the distal Facial Nerve and recording the CNAP from the nerve within the surgical field opposite to the normal direction of impulse conduction (antidromic recording) using a bipolar electrode (Richmond and Mahla, 1985). This technique was further updated by Colletti et al. (1996), using low-intensity stimulation of the mandibular branch of the Facial Nerve and monopolar recording techniques with an intracranial electrode. Methods based on CNAP rather than EMG recording have the advantage that they can be used even if muscle relaxants are used. Another advantage is that these methods allow actual continuous monitoring of Facial Nerve function, in contrast to EMG, which provides information only when the Facial Nerve is mechanically or electrically stimulated. On the other hand, the CNAP monitoring is less sensitive than EMG, and frequently requires averaging. Also, direct nerve monitoring is technically more difficult than EMG; attaching electrodes to delicate neural structures can be difficult and even injurious to the nerve. In addition, electrodes in the surgical field may limit exposure (Colletti et al., 1997).
Recording of F-waves from the nasal muscle is another method that made its way to clinical use in Facial Nerve monitoring (Wedekind and Klug, 1996). The F-wave is a late muscle potential that is believed to be due to recurrent discharge of a small percentage
($1–5%) of the motor neurons. It occurs due to antidromic spread of excitation that reaches the motor neurons and then again propagates orthodromically, producing a delayed muscle contraction. The intra- operative recording of an F-wave should reflect the integrity of the Facial Nerve pathway from the axon hillock to the motor end plate; a stable F-wave or reversible changes that occur during surgery usually indicate good postoperative Facial Nerve function (Wedekind and Klug, 2001). Although the F-wave may be a promising tool for intraoperative Facial Nerve monitoring, its use has been limited by its sensitivity to anesthesia and the fact that it may be normally absent in healthy adults.
Transcranial motor evoked potentials (tcMEP) is a new technique now widely used in spinal surgery. It is performed by electrical stimulation of the motor cortex with recording of EMG responses from appropriate muscles. This technique has been successfully adopted to monitor the Facial Nerve by multipulse stimulation of the contralateral motor cortex and recording of facial MEPs from the orbicularis oris muscle on the operative side. This method can provide an ongoing assessment of Facial Nerve function without the need of the surgeon to stimulate the nerve, and could be especially useful in cases in which the proximal FN is inaccessible to direct stimulation (Dong et al., 2005).
Another method under trial is the blink reflex, which is elicited by stimulation of the supraorbital nerve (trigeminal afferents) and consists of an early ipsilateral EMG response (R1) and a later bilateral response (R2), both mediated by the Facial Nerve (Kimura, 1989). It is the R1 response that is recorded intraoperatively. A main problem with blink reflex, how- ever, is the suppression of reflex activity by general anesthetics. It is possible to overcome this problem by greater reliance on intravenous anesthetics such as propofol together with the use of repetitive train stimulation. A chief advantage of the last two techniques is the ability to continuously monitor the Facial Nerve without the need for the surgeon to directly stimulate the nerve. Further improvement in Facial Nerve monitoring techniques are likely to come about when such methods of continuous assessment of Facial Nerve integrity become more refined and integrated.
|
Fig. 6. Illustrative examples of volume conducted activity on facial nerve (Facial Nerve) monitoring channels that can be mistaken for Facial Nerve responses. A: Upper trace shows response from temporalis muscle after stimulation of the trigeminal nerve; middle trace is the same response (trigeminal nerve stimulation) recorded simultaneously on the orbicularis oculi channel. Note the short latency of the two responses (3 ms). Lower trace shows a response to Facial Nerve stimulation on orbicularis oculi channel from the same patient (latency 6.2 ms). The trigeminal and Facial Nerve responses can be differentiated by their latency and the use of multiple channels. B: Responses on the orbicularis oris to stimulation of the nervus intermedius (upper) and Facial Nerve (lower). Nervus intermedius response can be recognized by its longer latency, smaller amplitude, and higher threshold for stimulation. C: A volume conducted response on the orbicularis oculi to stimulation of the abducens nerve (upper) compared to a Facial Nerve response recorded from the same muscle (lower). Note the lower amplitude and shorter latency of the response to abducens nerve stimulation; the onset of the response is not clearly demarcated from the stimulus artifact. The vertical lines indicate the point of stimulation.
|
References
Ashram, YA, Pitts, L, Jackler, R and Yingling, CD (2005) Intraoperative electrophysiological identification of the nervus intermedius. Otol. Neurotol., 26: 274–279.
Beck, DL, Atkins, JS Jr., Benecke, JE, Jr. and Brackmann, DE (1991) Intraoperative facial nerve monitoring: prog- nostic aspects during acoustic tumor removal. Otolaryn- gol. Head Neck Surg., 104: 780–782.
Blair, EA, Teeple, E Jr., Sutherland, RM, Shih, T and Chen, D (1994) Effect of neuromuscular blockade on facial nerve monitoring. Am. J. Otol., 15: 161–167.
Campbell, WW (1998) Generalized peripheral neuro- pathies. In: Essentials of Electrodiagnostic Medicine. Williams and Wilkins, Baltimore, MD, pp. 225–253.
Colletti, V, Fiorino, FG, Policante, Z and Bruni, L (1996) New perspectives in intraoperative facial nerve monitor- ing with antidromic potentials. Am. J. Otol., 17(5): 755–762.
Colletti, V, Fiorino, F, Policante, Z and Bruni, L (1997) Intraoperative monitoring of facial nerve antidromic potentials during acoustic neuroma surgery. Acta. Oto- laryngol. (Stockh), 117: 663–669.
Danke, JA and Wiegand, DA (1994) Investigation of a coaxial bipolar nerve stimulator for intraoperative motor nerve monitoring. Laryngoscope, 104: 619–622.
Dong, CC, MacDonald, DB, Akagami, R, Westerberg, B, Alkhani, A, Kanaan, I and Hassounah, M (2005) Intra- operative facial motor evoked potential monitoring with transcranial electrical stimulation during skull base sur- gery. Clin. Neurophysiol., 116: 588–596.
Ebersold, M, Harner, SG and Beatty, CW (1992) Current results of the retrosigmoid approach to acoustic neuri- noma. J. Neurosurg., 76: 901–909.
Filipo, R, De Seta, E and Bertoli, GA (2000) Intraoperative video monitoring of the facial nerve. Am. J. Otol., 21: 119–122.
Filipo, R, Pichi, B, Bertoli, GA and De Seta, E (2002) Video-based system for intraoperative facial nerve mon- itoring: comparison with electromyography. Otol. Neu- rotol., 23: 594–597.
Harper, CM and Daube, JR (1998) Facial nerve electromy- ography and other cranial nerve monitoring. J. Clin. Neu- rophysiol., 15: 206–216.
Kartush, JM and Bouchard, KR (1992) Intraoperative facial nerve monitoring in otology, neurotology, and skull base surgery. In: Neuromonitoring in Otology and Head and Neck Surgery. Raven Press, New York, pp. 99–120.
Kartush, JM, Niparko, JK, Bledsoe, SC, Graham, MD and Kemink, JL (1985) Intraoperative facial nerve monitor- ing: a comparison of stimulating electrodes. Laryngo- scope, 95: 1536–1540.
Kimura, J (1989) The blink reflex. In: J Kimura (Ed.), Elec- trodiagnosis in Diseases of Nerve and Muscle: Principles and Practice. F.A Davis Company, Philadelphia, 2nd ed., pp. 307–331.
Krause, F (1912) Surgery of the Brain and Spinal Cord. Rebman Company, New York, Vol. II.
Lalwani, AK, Butt, FY, Jackler, RK, Pitts, LH and Yingling, CD (1994) Facial nerve outcome after acoustic neuroma surgery: a study from the era of cranial nerve monitoring. Otolaryngol. Head Neck Surg., 111: 561–570.
Magliulo, G and Zardo, F (1997) Intra-operative facial nerve monitoring. Its predictive value after skull base surgery. J. Laryngol. Otol., 111: 715–718.
Mandpe, AH, Mikulec, A, Jackler, RK, Pitts, LH and Yingling, CD (1998) Comparison of responses amplitude versus stimulation threshold in predicting early postopera- tive facial nerve function after acoustic neuroma resection. Am. J. Otol., 19: 112–117.
May, M, Schaitkin, BM, Weit, RJ and Gupta, P (2000) Trauma to the facial nerve: external, surgical and iatro- genic. In: M May and BM Schaitkin (Eds.), The Facial Nerve. Thieme, New York, 2nd ed., pp. 373–382.
Mller, AR (1995) Monitoring of motor nerves. In: AR Mller (Ed.) Intraoperative Neurophysiologic Moni- toring. Harwood Academic Publishers, Sydney, pp. 173–216.
Monsell, EM (2005) Iatrogenic facial nerve injury: preven- tion and management. In: RK Jackler and DE Brackmann (Eds.), Neurotology. Elsevier Mosby, St. Louis, 2nd ed., pp. 1270–1278.
Nakao, Y, Piccirillo, E, Falcioni, M, Taibah, A, Kobayashi, T and Sanna, M (2001) Electromyographic evaluation of facial nerve damage in acoustic neuroma surgery. Otol. Neurotol., 22: 554–557.
Nuwer, MR, Daube, J, Fischer, C, Schramm, J and Yingling, CD (1993) Neuromonitoring during surgery. Report of an IFCN committee. J. Electroencephalogr. Clin. Neurophysiol., 87: 263–276.
Olivecrona, H (1940) Acoustic tumors. J. Neurol. Psychia- try, 3: 141–146.
Prasad, S, Hirsch, BE, Kamerer, DB, Durrant, J and Sekhar, LN (1993) Facial nerve function following cer- ebellopontine angle surgery: prognostic value of intra- operative thresholds. Am. J. Otol., 14: 330–333.
Prass, RL (1996) Iatrogenic facial nerve injury: the role of facial nerve monitoring. Otolaryngol. Clin. North Am., 29: 265–275.
Prass, RL and Lu ̈ders, H (1986) Acoustic (loudspeaker) facial electromyographic monitoring: Part 1. Evoked electromyographic activity during acoustic neuroma resection. Neurosurgery, 19: 392–400.
Prass, RL, Kinney, SE, Hardy, RW, Jr., Hahn, JF and Luders, H (1987) Acoustic (loudspeaker) facial EMG monitoring: II. Use of evoked EMG activity during acoustic neuroma resection. Otolaryngol. Head Neck Surg., 97: 541–551.
Preston, DC and Shapiro, BE (1998) Fundamentals of nerve conduction studies: basic nerve conduction studies. In: DC Preston and BE Shapiro (Eds.), Electromyography and Neuromuscular Disorders. Clinical Electrophysio- logic Correlations. Butterworth-Heinemann, Boston, MA, pp. 25–44.
Pool, JL (1966) Suboccipital surgery for acoustic neurino- mas: advantages and disadvantages. J. Neurosurg., 24: 483–492.
Richmond, IL and Mahla, M (1985) Use of antidromic recording to monitor facial nerve function intraopera- tively. Neurosurgery, 16: 458–462.
Robinson, AJ (1995) Physiology of muscle and nerve. In: AJ Robinson and L Snyder-Mackler (Eds.), Clinical Electro- physiology: Electrotherapy and Electrophysiologic Test- ing. Williams and Wilkins, Baltimore, MD, pp. 110–117.
Romstock, J, Strauss, C and Fahlbusch, R (2000) Continu- ous electromyography monitoring of motor cranial nerves during cerebellopontine angle surgery. J. Neuro- surg., 93: 586–593.
Silverstein, H, Willcox, TO, Jr., Rosenberg, SI and Seid- man, MD (1994) Prediction of facial nerve function fol- lowing acoustic neuroma resection using intraoperative facial nerve stimulation. Laryngoscope, 104: 539–544.
Taha, JM, Tew, JM and Keith, RW (1995) Proximal to dis- tal facial amplitude ratios as predictors of facial nerve function after acoustic neuroma excision. J. Neurosurg., 83: 994–998.
Wedekind, C and Klug, N (1996) F-wave recordings from nasal muscle for intraoperative monitoring of facial nerve function. Zentralbl. Neurochir., 57: 184–189.
Wedekind, C and Klug, N (2001) Recording nasal muscle F waves and electromyographic activity of the facial muscles: a comparison of two methods used for intra- operative monitoring of facial nerve function. J. Neuro- surg., 95: 974–978.
Yingling, CD and Ashram, YA (2005a) Intraoperative monitoring of cranial nerves in skull base surgery. In: RK Jackler and DE Brackmann (Eds.), Neurotology. Elsevier Mosby, St. Louis, pp. 958–993.
Yingling, CD and Ashram, YA (2005b) Intraoperative mon- itoring of cranial nerves in Neurotologic Surgery. In: CW Cummings, PW Flint, LA Harker, BH Haughey, MA Richardson, KH Robbins, DE Schuller and JR Thomas (Eds.), Otolaryngology Head and Neck Surgery. Elsevier Mosby, St. Louis, 4th ed., pp. 3877–3910.
Yingling, CD and Gardi, JN (1992) Intraoperative monitor- ing of facial and cochlear nerves during acoustic neuroma surgery. Otolaryngol. Clin. North Am., 25: 413–448.
York, D (1992) Basic principles of neurophysiologic record- ings in the operating room. In: J Kartush and K Bouchard (Eds.), Neuromonitoring in Otology and Head and Neck Surgery. Raven Press, New York, pp. 1–19.
Zeitouni, AG, Hammerschlag, PE and Cohen, NL (1997) Prognostic significance of intraoperative facial nerve stimulus thresholds. Am. J. Otol., 18: 494–497.
Beck, DL, Atkins, JS Jr., Benecke, JE, Jr. and Brackmann, DE (1991) Intraoperative facial nerve monitoring: prog- nostic aspects during acoustic tumor removal. Otolaryn- gol. Head Neck Surg., 104: 780–782.
Blair, EA, Teeple, E Jr., Sutherland, RM, Shih, T and Chen, D (1994) Effect of neuromuscular blockade on facial nerve monitoring. Am. J. Otol., 15: 161–167.
Campbell, WW (1998) Generalized peripheral neuro- pathies. In: Essentials of Electrodiagnostic Medicine. Williams and Wilkins, Baltimore, MD, pp. 225–253.
Colletti, V, Fiorino, FG, Policante, Z and Bruni, L (1996) New perspectives in intraoperative facial nerve monitor- ing with antidromic potentials. Am. J. Otol., 17(5): 755–762.
Colletti, V, Fiorino, F, Policante, Z and Bruni, L (1997) Intraoperative monitoring of facial nerve antidromic potentials during acoustic neuroma surgery. Acta. Oto- laryngol. (Stockh), 117: 663–669.
Danke, JA and Wiegand, DA (1994) Investigation of a coaxial bipolar nerve stimulator for intraoperative motor nerve monitoring. Laryngoscope, 104: 619–622.
Dong, CC, MacDonald, DB, Akagami, R, Westerberg, B, Alkhani, A, Kanaan, I and Hassounah, M (2005) Intra- operative facial motor evoked potential monitoring with transcranial electrical stimulation during skull base sur- gery. Clin. Neurophysiol., 116: 588–596.
Ebersold, M, Harner, SG and Beatty, CW (1992) Current results of the retrosigmoid approach to acoustic neuri- noma. J. Neurosurg., 76: 901–909.
Filipo, R, De Seta, E and Bertoli, GA (2000) Intraoperative video monitoring of the facial nerve. Am. J. Otol., 21: 119–122.
Filipo, R, Pichi, B, Bertoli, GA and De Seta, E (2002) Video-based system for intraoperative facial nerve mon- itoring: comparison with electromyography. Otol. Neu- rotol., 23: 594–597.
Harper, CM and Daube, JR (1998) Facial nerve electromy- ography and other cranial nerve monitoring. J. Clin. Neu- rophysiol., 15: 206–216.
Kartush, JM and Bouchard, KR (1992) Intraoperative facial nerve monitoring in otology, neurotology, and skull base surgery. In: Neuromonitoring in Otology and Head and Neck Surgery. Raven Press, New York, pp. 99–120.
Kartush, JM, Niparko, JK, Bledsoe, SC, Graham, MD and Kemink, JL (1985) Intraoperative facial nerve monitor- ing: a comparison of stimulating electrodes. Laryngo- scope, 95: 1536–1540.
Kimura, J (1989) The blink reflex. In: J Kimura (Ed.), Elec- trodiagnosis in Diseases of Nerve and Muscle: Principles and Practice. F.A Davis Company, Philadelphia, 2nd ed., pp. 307–331.
Krause, F (1912) Surgery of the Brain and Spinal Cord. Rebman Company, New York, Vol. II.
Lalwani, AK, Butt, FY, Jackler, RK, Pitts, LH and Yingling, CD (1994) Facial nerve outcome after acoustic neuroma surgery: a study from the era of cranial nerve monitoring. Otolaryngol. Head Neck Surg., 111: 561–570.
Magliulo, G and Zardo, F (1997) Intra-operative facial nerve monitoring. Its predictive value after skull base surgery. J. Laryngol. Otol., 111: 715–718.
Mandpe, AH, Mikulec, A, Jackler, RK, Pitts, LH and Yingling, CD (1998) Comparison of responses amplitude versus stimulation threshold in predicting early postopera- tive facial nerve function after acoustic neuroma resection. Am. J. Otol., 19: 112–117.
May, M, Schaitkin, BM, Weit, RJ and Gupta, P (2000) Trauma to the facial nerve: external, surgical and iatro- genic. In: M May and BM Schaitkin (Eds.), The Facial Nerve. Thieme, New York, 2nd ed., pp. 373–382.
Mller, AR (1995) Monitoring of motor nerves. In: AR Mller (Ed.) Intraoperative Neurophysiologic Moni- toring. Harwood Academic Publishers, Sydney, pp. 173–216.
Monsell, EM (2005) Iatrogenic facial nerve injury: preven- tion and management. In: RK Jackler and DE Brackmann (Eds.), Neurotology. Elsevier Mosby, St. Louis, 2nd ed., pp. 1270–1278.
Nakao, Y, Piccirillo, E, Falcioni, M, Taibah, A, Kobayashi, T and Sanna, M (2001) Electromyographic evaluation of facial nerve damage in acoustic neuroma surgery. Otol. Neurotol., 22: 554–557.
Nuwer, MR, Daube, J, Fischer, C, Schramm, J and Yingling, CD (1993) Neuromonitoring during surgery. Report of an IFCN committee. J. Electroencephalogr. Clin. Neurophysiol., 87: 263–276.
Olivecrona, H (1940) Acoustic tumors. J. Neurol. Psychia- try, 3: 141–146.
Prasad, S, Hirsch, BE, Kamerer, DB, Durrant, J and Sekhar, LN (1993) Facial nerve function following cer- ebellopontine angle surgery: prognostic value of intra- operative thresholds. Am. J. Otol., 14: 330–333.
Prass, RL (1996) Iatrogenic facial nerve injury: the role of facial nerve monitoring. Otolaryngol. Clin. North Am., 29: 265–275.
Prass, RL and Lu ̈ders, H (1986) Acoustic (loudspeaker) facial electromyographic monitoring: Part 1. Evoked electromyographic activity during acoustic neuroma resection. Neurosurgery, 19: 392–400.
Prass, RL, Kinney, SE, Hardy, RW, Jr., Hahn, JF and Luders, H (1987) Acoustic (loudspeaker) facial EMG monitoring: II. Use of evoked EMG activity during acoustic neuroma resection. Otolaryngol. Head Neck Surg., 97: 541–551.
Preston, DC and Shapiro, BE (1998) Fundamentals of nerve conduction studies: basic nerve conduction studies. In: DC Preston and BE Shapiro (Eds.), Electromyography and Neuromuscular Disorders. Clinical Electrophysio- logic Correlations. Butterworth-Heinemann, Boston, MA, pp. 25–44.
Pool, JL (1966) Suboccipital surgery for acoustic neurino- mas: advantages and disadvantages. J. Neurosurg., 24: 483–492.
Richmond, IL and Mahla, M (1985) Use of antidromic recording to monitor facial nerve function intraopera- tively. Neurosurgery, 16: 458–462.
Robinson, AJ (1995) Physiology of muscle and nerve. In: AJ Robinson and L Snyder-Mackler (Eds.), Clinical Electro- physiology: Electrotherapy and Electrophysiologic Test- ing. Williams and Wilkins, Baltimore, MD, pp. 110–117.
Romstock, J, Strauss, C and Fahlbusch, R (2000) Continu- ous electromyography monitoring of motor cranial nerves during cerebellopontine angle surgery. J. Neuro- surg., 93: 586–593.
Silverstein, H, Willcox, TO, Jr., Rosenberg, SI and Seid- man, MD (1994) Prediction of facial nerve function fol- lowing acoustic neuroma resection using intraoperative facial nerve stimulation. Laryngoscope, 104: 539–544.
Taha, JM, Tew, JM and Keith, RW (1995) Proximal to dis- tal facial amplitude ratios as predictors of facial nerve function after acoustic neuroma excision. J. Neurosurg., 83: 994–998.
Wedekind, C and Klug, N (1996) F-wave recordings from nasal muscle for intraoperative monitoring of facial nerve function. Zentralbl. Neurochir., 57: 184–189.
Wedekind, C and Klug, N (2001) Recording nasal muscle F waves and electromyographic activity of the facial muscles: a comparison of two methods used for intra- operative monitoring of facial nerve function. J. Neuro- surg., 95: 974–978.
Yingling, CD and Ashram, YA (2005a) Intraoperative monitoring of cranial nerves in skull base surgery. In: RK Jackler and DE Brackmann (Eds.), Neurotology. Elsevier Mosby, St. Louis, pp. 958–993.
Yingling, CD and Ashram, YA (2005b) Intraoperative mon- itoring of cranial nerves in Neurotologic Surgery. In: CW Cummings, PW Flint, LA Harker, BH Haughey, MA Richardson, KH Robbins, DE Schuller and JR Thomas (Eds.), Otolaryngology Head and Neck Surgery. Elsevier Mosby, St. Louis, 4th ed., pp. 3877–3910.
Yingling, CD and Gardi, JN (1992) Intraoperative monitor- ing of facial and cochlear nerves during acoustic neuroma surgery. Otolaryngol. Clin. North Am., 25: 413–448.
York, D (1992) Basic principles of neurophysiologic record- ings in the operating room. In: J Kartush and K Bouchard (Eds.), Neuromonitoring in Otology and Head and Neck Surgery. Raven Press, New York, pp. 1–19.
Zeitouni, AG, Hammerschlag, PE and Cohen, NL (1997) Prognostic significance of intraoperative facial nerve stimulus thresholds. Am. J. Otol., 18: 494–497.